Publications
See the Google Scholar version here.
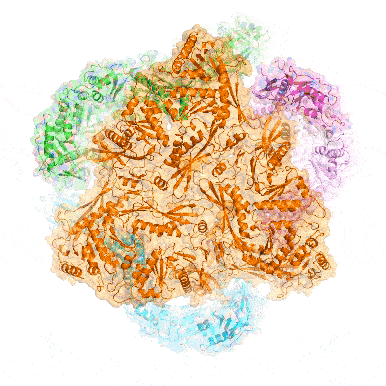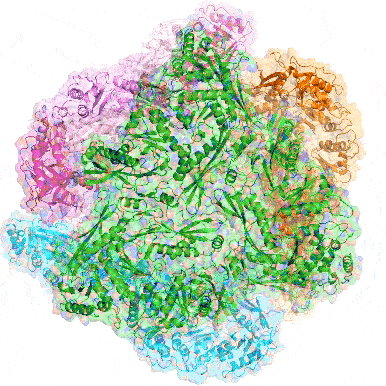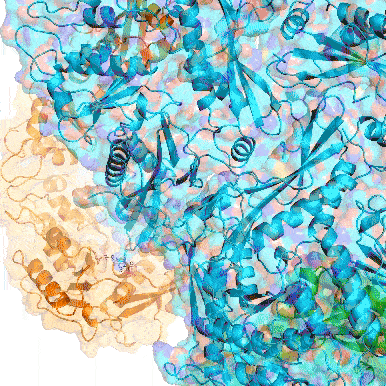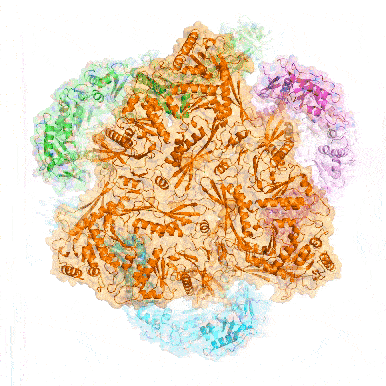
Point mutation in a virus-like capsid drives symmetry reduction to form tetrahedral cages
T. N. Szyszka, M. P. Andreas, F. Lie, L. M. Miller, L. S. R. Adamson, F. Fatehi, R. Twarock, B. E. Draper, M. F. Jarrold, T. W. Giessen, Y. H. Lau
Proc. Natl. Acad. Sci. USA 2024, 121, e2321260121.
doi: 10.1073/pnas.2321260121
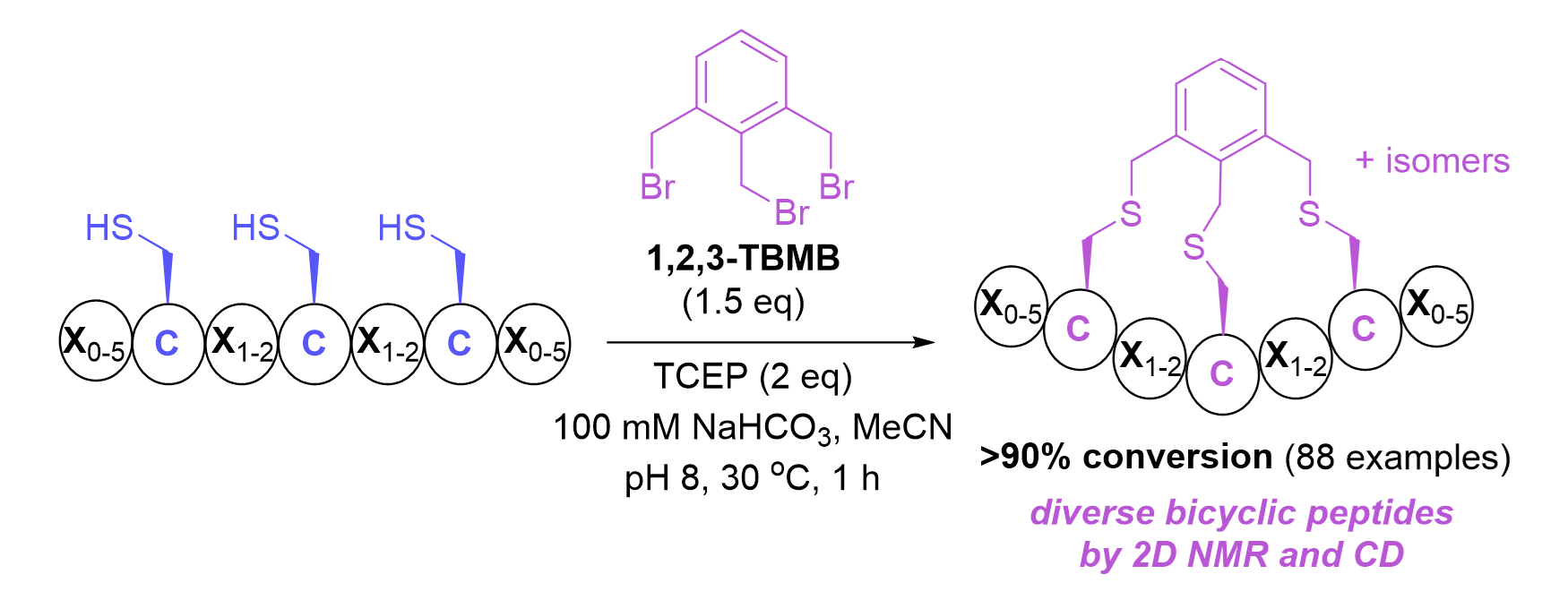
Accessing diverse bicyclic peptide conformations using 1,2,3-TBMB as a linker
H. K. Sudhakar, J. T. K. Yau, L. J. Alcock, Y. H. Lau
Org. Biomol. Chem. 2024, 22, 6095-6102.doi: 10.1039/D4OB00901K
Fluorescence polarization assay for screening FANCM-RMI inhibitors to target the alternative lengthening of telomeres
L. J. Alcock, H. K. Sudhakar, R. Young, Y. H. Lau
Meth. Enzymol. 2024, 698, 361-378. doi: 10.1016/bs.mie.2024.04.014
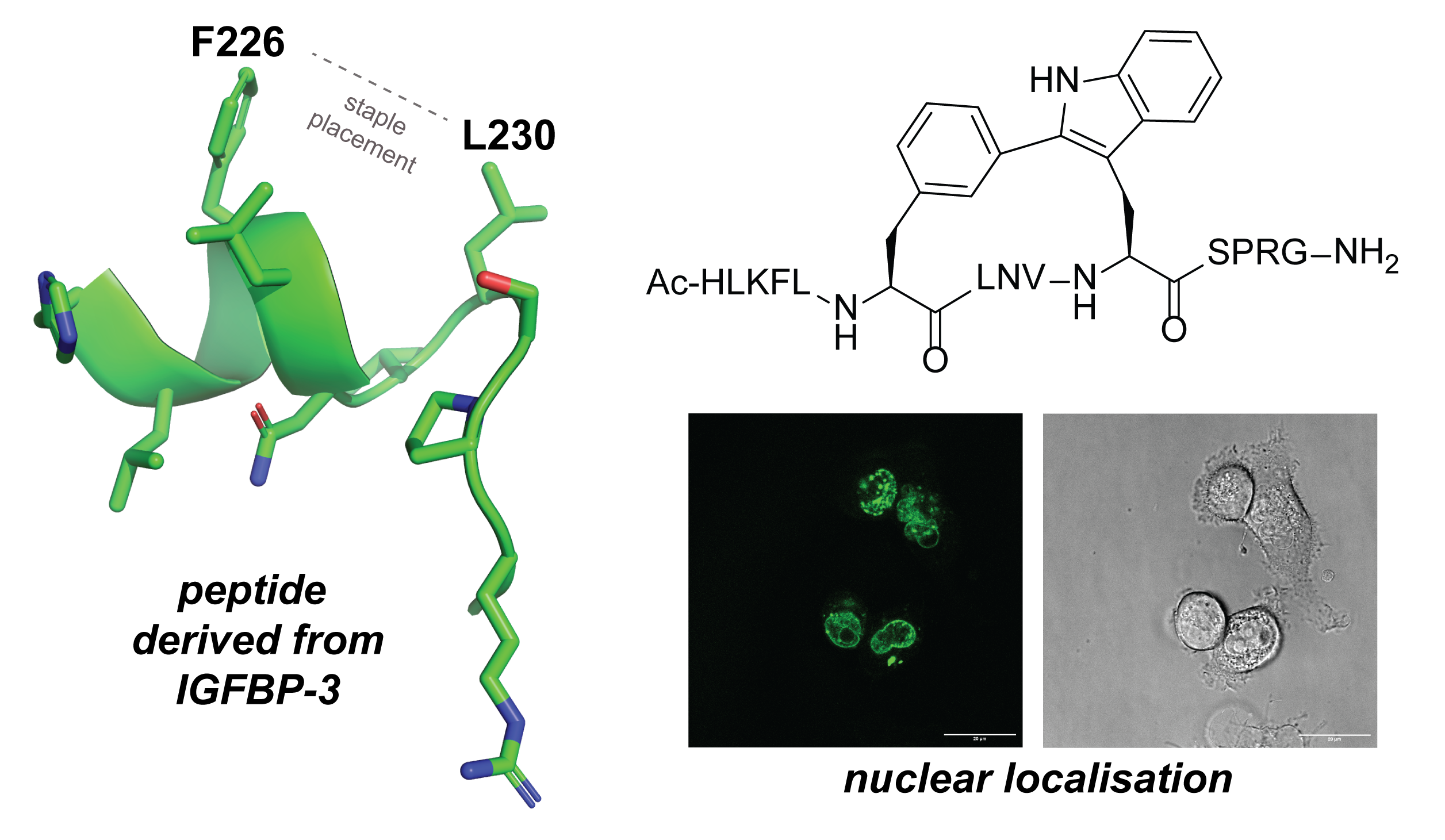
Development of stapled NONO-associated peptides reveals unexpected cell permeability and nuclear localisation
R. Young, T. Huang, Z. Luo, Y. S. Tan, A. Kaur, Y. H. Lau
J. Pept. Sci. 2024, 30, e3562. doi: 10.1002/psc.3562
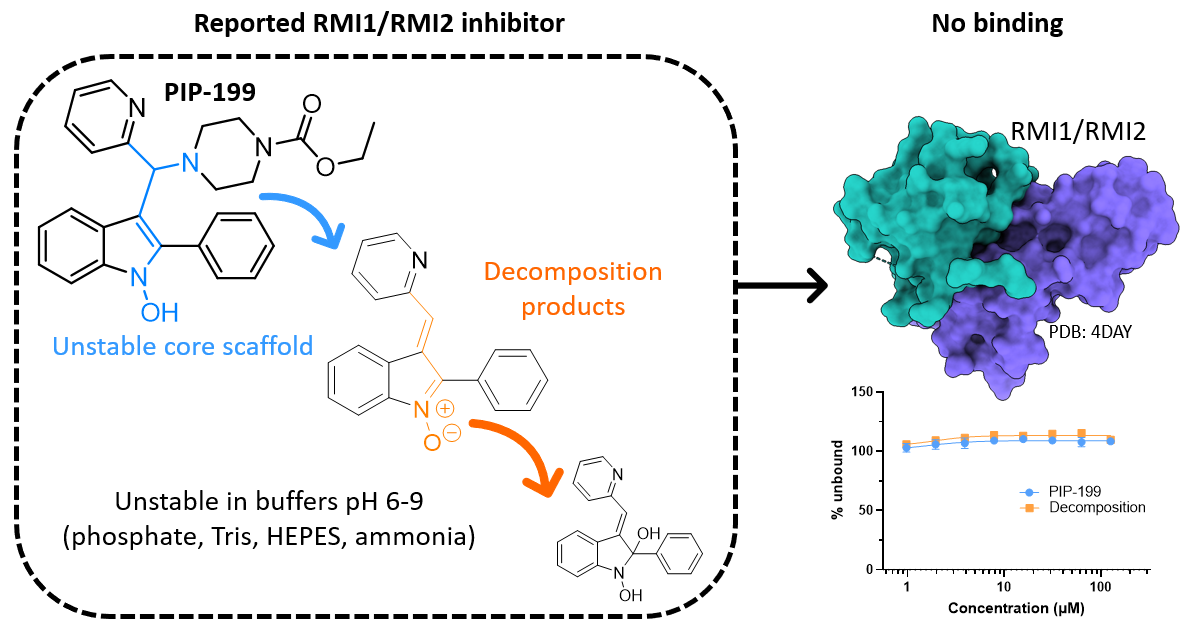
Mannich base PIP-199 is a chemically unstable pan-assay interference compound
X. Wu, H. K. Sudhakar, L. Alcock, Y. H. Lau
J. Med. Chem. 2023, 66, 11271-11281. doi: 10.1021/acs.jmedchem.3c00674
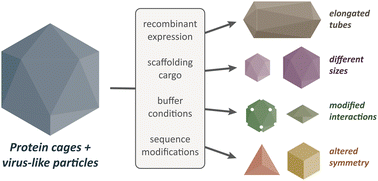
Structural polymorphism in protein cages and virus-like particles
F. Lie, T. N. Szyszka, Y. H. Lau
J. Mater. Chem. B 2023, 11, 6516-6526.doi: 10.1039/D3TB00991B
The supramolecular chemistry of protein cages and viruses
Y. H. Lau
Aust. J. Chem. 2023, 76, 671-676. doi: 10.1071/CH23102
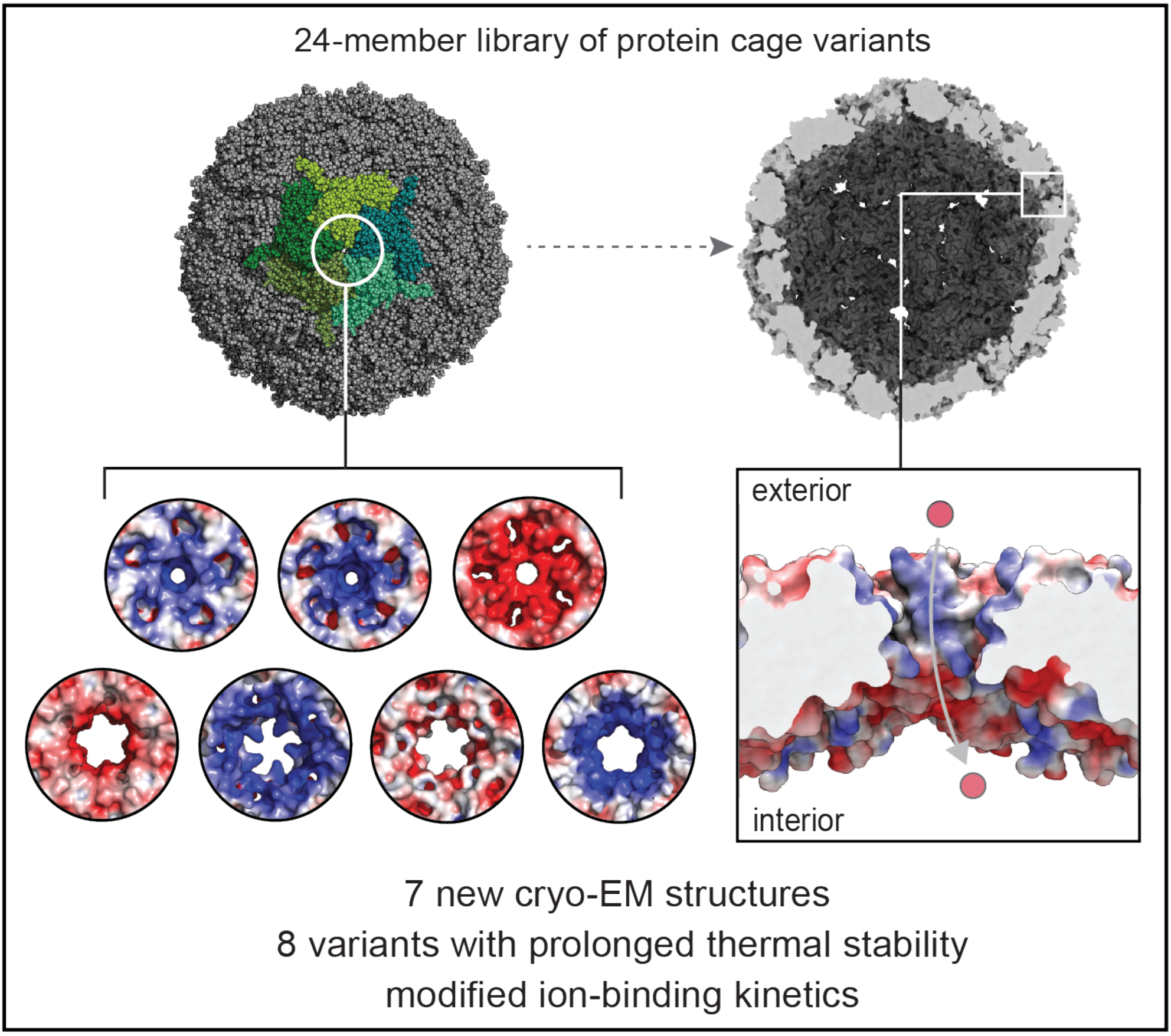
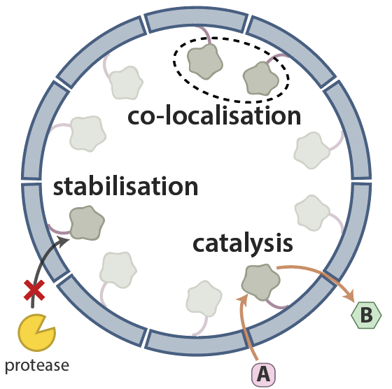
Pore structure controls stability and molecular flux in engineered protein cages
L. S. R. Adamson, N. Tasneem, M. P. Andreas, W. Close, T. N. Szyszka, E. Jenner, R. Young, L. C. Cheah, A. Norman, F. Sainsbury, T. W. Giessen*, Y. H. Lau*
Science Advances 2022, 8, abl7346. doi: 10.1126/sciadv.abl7346
Featured by Microscopy Australia: https://micro.org.au/news/synthetic-biology-could-boost-biomanufacturing-efficiency/
How pore architecture regulates the function of nanoscale protein compartments
N. Tasneem, T. N. Szyszka, E. N. Jenner, Y. H. Lau
ACS Nano 2022, 16, 8540-8556. doi:10.1021/acsnano.2c02178
Molecular display on protein nanocompartments: design strategies and systems applications
T. N. Szyszka, E. N. Jenner, N. Tasneem, Y. H. Lau
ChemSystemsChem 2022, 4, e202100025. doi: 10.1002/syst.202100025
Selected as journal cover and featured with an author profile: doi:10.1002/syst.202100043
Encapsulin nanocompartments for biomanufacturing applications
T. N. Szyszka, L. S. R. Adamson, Y. H. Lau
Microbial Production of High-Value Products. Microbiology monographs 2022, 37, 309-334. doi:10.1007/978-3-031-06600-9_12
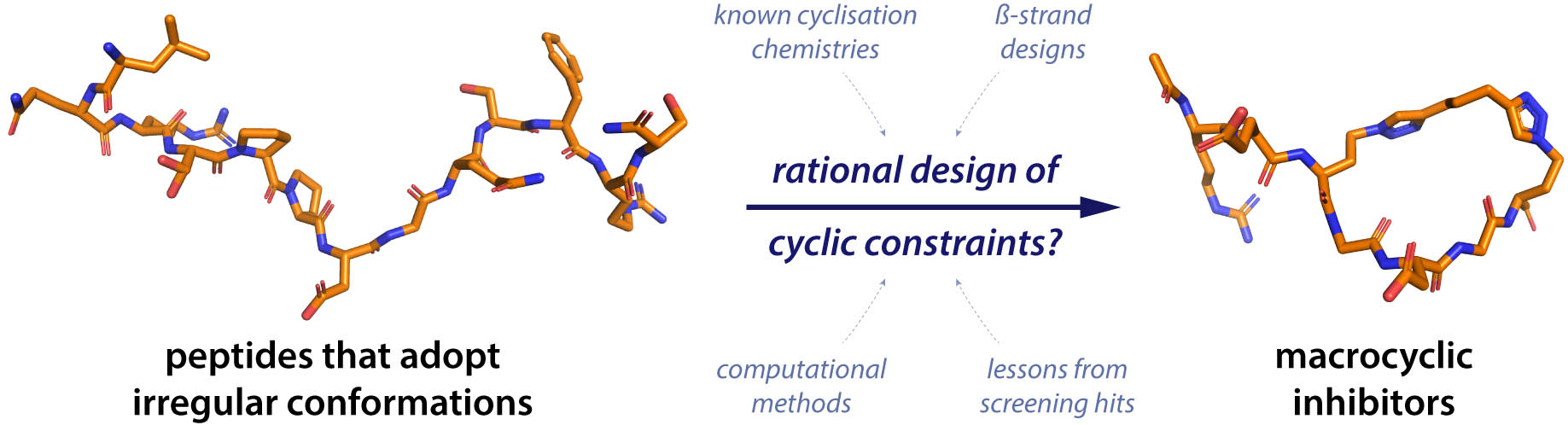
Cyclisation strategies for stabilising peptides with irregular conformations
Q. N. Vu, R. Young, H. K. Sudhakar, T. Gao, T. Huang, Y. S. Tan, Y. H. Lau
RSC Med. Chem. 2021, 12, 887-901. doi: 10.1039/D1MD00098E
An artificial self-assembling nanocompartment for organising metabolic pathways in yeast
L. C. Cheah, T. Stark, L. S. R. Adamson, R. S. Abidin, Y. H. Lau, F. Sainsbury, C. E. Vickers
ACS Synth. Biol. 2021, 10, 3251-3263. doi: 10.1021/acssynbio.1c00045
Functionalized double strain-promoted stapled peptides for inhibiting the p53-MDM2 interaction
K. Sharma, A. V. Strizhak, E. Fowler, W. Xu, B. Chappell, H. F. Sore, W. R. J. D. Galloway, M. N. Grayson*, Y. H. Lau*, L. S. Itzhaki*, D. R. Spring*
ACS Omega 2020, 5, 1157-1169. doi: 10.1021/acsomega.9b03459
Water-soluble, stable and azide-reactive strained dialkynes for biocompatible double strain-promoted click chemistry
K. Sharma, A. V. Strizhak, E. Fowler, X. Wang, W. Xu, C. H. Jensen, Y. Wu, H. F. Sore, Y. H. Lau*, M. Hyvönen*, L. S. Itzhaki*, D. R. Spring*
Org. Biomol. Chem. 2019, 17, 8014-8018. doi: 10.1039/C9OB01745C
Toolbox of diverse linkers for navigating the cellular efficacy landscape of stapled peptides
Y. Wu, A. Kaur, E. Fowler, M. M. Wiedmann, R. Young, W. R. J. D. Galloway, L. Olsen, H. F. Sore, A. Chattopadhyay, T. T.-L. Kwan, W. Xu, S. J. Walsh, P. de Andrade, M. Janecek, S. Arumugam, L. S. Itzhaki, Y. H. Lau*, D. R. Spring*
ACS Chem. Biol. 2019, 14, 526-533. doi: 10.1021/acschembio.9b00063
Prokaryotic nanocompartments form synthetic organelles in a eukaryote
Y. H. Lau, T. W. Giessen, W. J. Altenburg, P. A. Silver
Nat. Commun. 2018, 9, 1311. doi: 10.1038/s41467-018-03768-x
Stapled peptides as a new technology to investigate protein-protein interactions in human platelets
J. Iegre, N. S. Ahmed, J. S. Gaynord, Y. Wu, K. M. Herlihy, Y. S. Tan, M. E. Lopes-Pires, R. Jha, Y. H. Lau, H. F. Sore, C. Verma, D. H. O’Donvan, N. Pugh, D. R. Spring
Chem. Sci. 2018, 9, 4638-4643. doi: 10.1039/C8SC00284C
Molecular switches for any pH: a systematic study of the versatile coordination behaviour of cyclam scorpionands
Y. H. Lau, J. K. Clegg, J. R. Price, R. B. Marquart, M. H. Todd, P. J. Rutledge
Chem. Eur. J. 2018, 24, 1573-1585. doi: 10.1002/chem.201703488
Synthetic genome recoding: new genetic codes for new features
J. Kuo, F. Stirling, Y. H. Lau, Y. Shulgina, J. C. Way, P. A. Silver
Curr. Genet. 2018, 64, 327-333. doi: 10.1007/s00294-017-0754-z
Double click stapled peptides for inhibiting protein-protein interactions
K. Sharma, D. L. Kunciw, W. Xu, M. M. Wiedmann, Y. Wu, H. F. Sore, W. R. J. D. Galloway, Y. H. Lau, L. S. Itzhaki, D. R. Spring
Cyclic Peptides: From Bioorganic Synthesis to Applications 2018, ISBN 978-1-78262-528-5, 164-187. doi: 10.1039/9781788010153-00164
Targeting the genome-stability hub Ctf4 by stapled-peptide design
Y. Wu, F. Villa, J. Maman, Y. H. Lau, L. Dobnikar, A. C. Simon, K. Labib, D. R. Spring, L. Pellegrini
Angew. Chem. Int. Ed. 2017, 56, 12866-12872. doi: 10.1002/anie.201705611
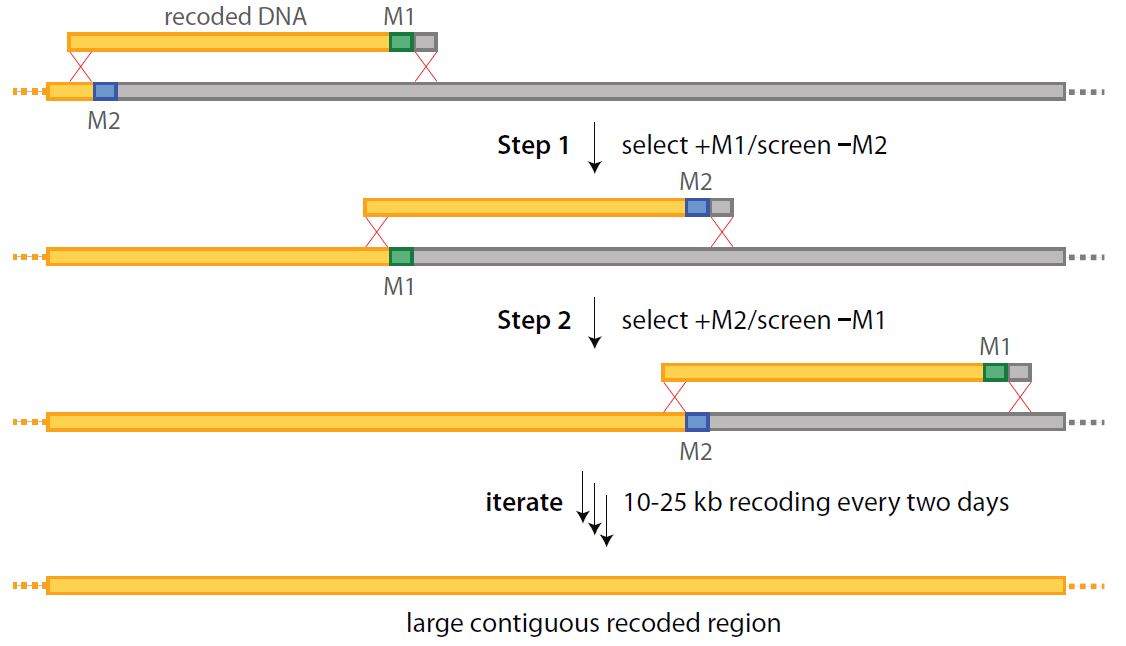
Large-scale recoding of a bacterial genome by iterative recombineering of synthetic DNA
Y. H. Lau, F. Stirling, J. Kuo, M. A. P. Karrenbelt, Y. A. Chan, A. Riesselman, C. A. Horton, E. Schäfer, D. Lips, M. T. Weinstock, D. G. Gibson, J. C. Way, P. A. Silver
Nucleic Acids Res. 2017, 45, 6971-6980. doi: 10.1093/nar/gkx415
Macrocyclized extended peptides: inhibiting the substrate-recognition domain of tankyrase
W. Xu, Y. H. Lau, G. Fischer, Y. S. Tan, A. Chattopadhyay, M. de la Roche, M. Hyvönen, C. Verma, D. Spring, L. Itzhaki
J. Am. Chem. Soc. 2017, 139, 2245-2256. doi: 10.1021/jacs.6b10234
Development of a multifunctional benzophenone linker for peptide stapling and photoaffinity labelling
Y. Wu, L. B. Olsen, Y. H. Lau, C. H. Jensen, M. Rossmann, Y. R. Baker, H. F. Sore, S. Collins, D. R. Spring
ChemBioChem 2016, 17, 689-692. doi: 10.1002/cbic.201500648
Double strain-promoted macrocyclization for the rapid selection of cell-active stapled peptides
Y. H. Lau, Y. Wu, M. Rossmann, B. X. Tan, P. de Andrade, Y. S. Tan, C. Verma, G. J. McKenzie, A. R. Venkitaraman, M. Hyvönen, D. R. Spring
Angew. Chem. Int. Ed. 2015, 54, 15410-15413. doi: 10.1002/anie.201508416
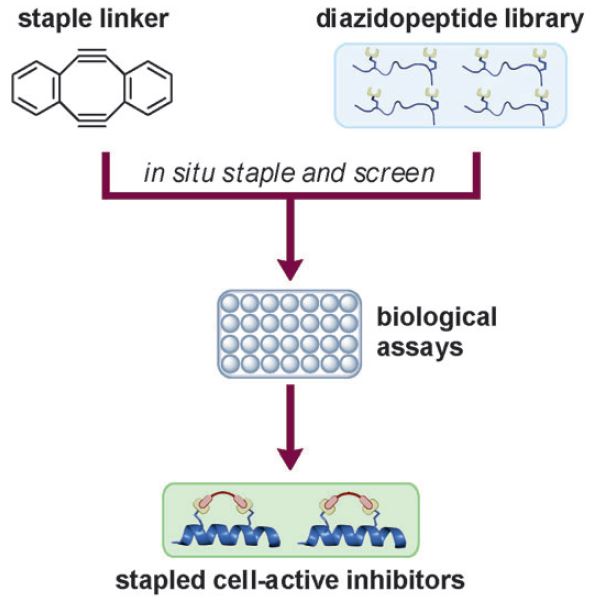
A two-component ‘double-click’ approach to peptide stapling
Y. H. Lau, Y. Wu, P. de Andrade, W. R. J. D. Galloway, D. R. Spring
Nat. Protoc. 2015, 10, 585-594. doi: 10.1038/nprot.2015.033
Peptide stapling techniques based on different macrocyclisation chemistries
Y. H. Lau, P. de Andrade, Y. Wu, D. R. Spring
Chem. Soc. Rev. 2015, 44, 91-102. doi: 10.1039/c4cs00246f
Linear aliphatic dialkynes as alternative linkers for double-click stapling of p53-derived peptides
Y. H. Lau, P. de Andrade, G. J. McKenzie, A. R. Venkitaraman, D. R. Spring
ChemBioChem 2014, 15, 2680-2683. doi: 10.1002/cbic.201402374
Investigating peptide sequence variations for 'double-click' stapled p53 peptides
Y. H. Lau, P. de Andrade, N. Sköld, G. J. McKenzie, A. R. Venkitaraman, C. Verma, D. P. Lane, D. R. Spring
Org. Biomol. Chem. 2014, 12, 4074-4077. doi: 10.1039/c4ob00742e
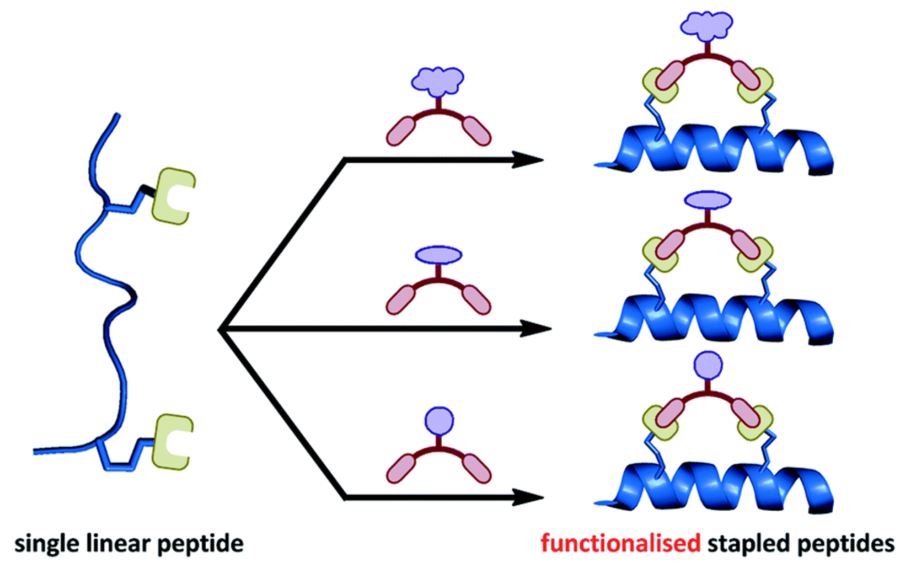
Functionalised staple linkages for modulating the cellular activity of stapled peptides
Y. H. Lau, P. de Andrade, S.-T. Quah, M. Rossmann, L. Laraia, N. Sköld, T. J. Sum, P. J. E. Rowling, T. L. Joseph, C. Verma, M. Hyvönen, L. S. Itzhaki, A. R. Venkitaraman, C. J. Brown, D. P. Lane, D. R. Spring
Chem. Sci. 2014, 5, 1804-1809. doi: 10.1039/c4sc00045e
Efficient synthesis of Fmoc-protected azido amino acids
Y. H. Lau, D. R. Spring
Synlett 2011, 1917-1919. doi: 10.1055/s-0030-1260950
A click fluorophore sensor can distinguish Cu(II) and Hg(II) via selective anion-induced demetallation
Y. H. Lau, J. Price, M. H. Todd, P. J. Rutledge
Chem. Eur. J. 2011, 17, 2850-2858. doi: 10.1002/chem.201002477
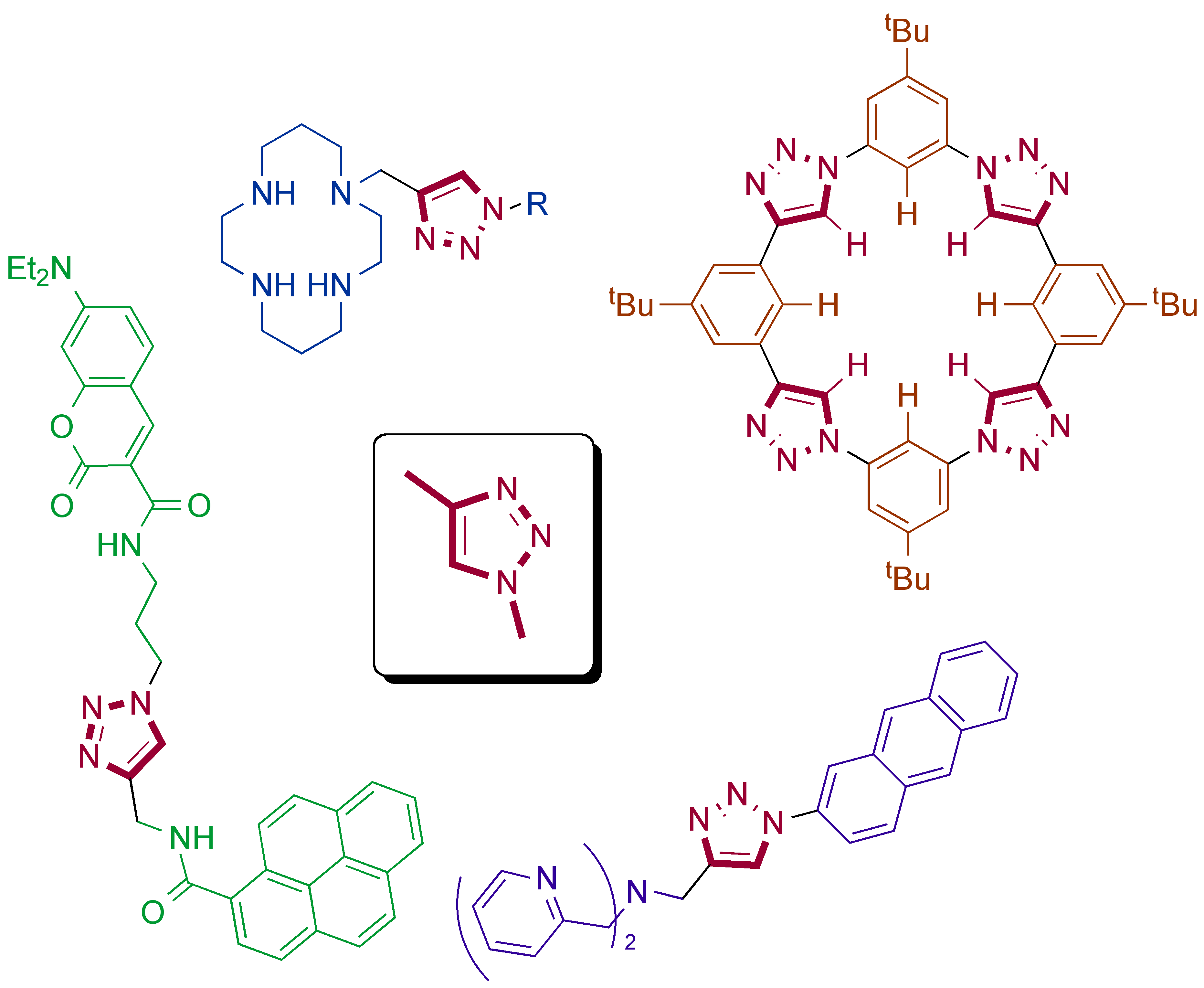
Chemical sensors that incorporate click-derived triazoles
Y. H. Lau, P. J. Rutledge, M. Watkinson, M. H. Todd
Chem. Soc. Rev. 2011, 40, 2848-2866. doi: 10.1039/c0cs00143k
Synthesis, electrochemistry and metal binding properties of monosubstituted ferrocenoyl peptides with thioether-containing sidechains
C. C. G. Scully, Y. H. Lau, P. Jensen, P. J. Rutledge
J. Organomet. Chem. 2011, 696, 715-721. doi: 10.1016/j.jorganchem.2010.09.056